Gallery
Water, energy and surfaces: an unexpectedly profound linkage
2009 г.
Pollack Gerald H.
Department of Bioengineering Box 355061
University of Washington, Seattle WA 98195
For most scientists, water is fairly uninteresting. Especially in biology, water is considered merely a background carrier of the more interesting molecules in life — a largely neutral entity that bears responsibility mainly for suspending the molecules of more central interest. Likewise in the physical sciences, except for the few molecular layers immediately adjacent to surfaces and except for situations where water is the central protagonist, such as atmospheric sciences, water is considered to play only a secondary role in most physical or chemical processes.
Contrastingly, we have found evidence that water plays an unexpectedly profound role — one that may inevitably impact practically all phenomena that take place in aqueous solution.
Recent data from this laboratory show that in the vicinity of common hydrophilic materials, water molecules become ordered. Properties of this ordered zone — mechanical, chemical and optical — all differ from those of bulk water. These findings stem from an original core observation: colloids and solutes are profoundly excluded from the region next to hydrophilic materials. The exclusion zone (EZ) commonly extends up to hundreds of micrometers from the surface (see figure to right).
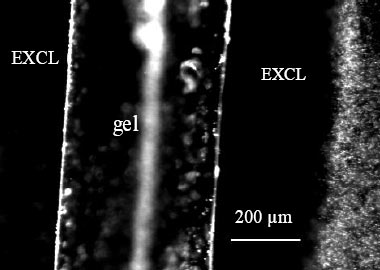
Solute exclusion (EXCL) in the vicinity of polyacrylic acid gel. Gel
runs vertically. The blurred vertical white element to the right of
“gel” is an optical artifact. The gel was placed on a coverslip,
superfused with a suspension of 1-μm carboxylate-coated microspheres,
and observed in an inverted microscope equipped with a 20x objective.
Image obtained 20 min. after superfusion. Microspheres (seen on right
edge) undergo active thermal motion, but do not enter the exclusion
zone.
The core findings are detailed in two papers Zheng and Pollack (Phys Rev E.: 68: 031408, 2003) and Zheng et al. (Adv. Colloid Interface Sci. 127: 19-27, 2006). More easily accessible is the link to a public lecture <http://uwtv.org/programs/displayevent.aspx?rID=22222> delivered as recipient of the 2008 University of Washington Annual Lectureship Award. This lecture describes the evidence as well as the implications in a manner easily accessible for non-experts.
The first of the two cited papers deals with the question of whether the finding of long-range exclusion of solutes may have some trivial explanation. Plausible candidates were ruled out by an extensive series of controls. Since then, at least a dozen groups worldwide have informally tested and confirmed the basic finding. In fact, a similar result had been published four decades ago: in addressing the origin of the so-called “unstirred layer” adjacent to biological tissues, a region where mixing is known to be extremely slow, Green and Otori (J. Physiol. London, 207:93-102 1970) showed in both corneal tissue and contact lenses (polyHEMA) that the unstirred layer excludes microspheres; exclusion zones several hundred micrometers wide were found — essentially the same has we have found in our studies. Hence, there is little question of the existence of unexpectedly large exclusion zones next to hydrophilic surfaces. The question is what such zones might mean.
The second Zheng paper (2006) shows that the physical chemical properties of the EZ differ from those of bulk water. Four sets of results are presented:
- NMR rotational relaxation time in the EZ is shorter than in bulk water;
- Infrared radiation from the EZ is less than from bulk water;
- Potential gradients exist in the EZ, but not in bulk water;
- UV-Vis absorption spectra are markedly different in the EZ.
Two additional features have recently been added. They are:
- Polarizing microscopy shows that the EZ is birefringent, confirming molecular ordering in that zone;
- Falling-ball viscometry shows that EZ water is considerably more viscous than bulk water.
Hence, six independent approaches show that EZ water differs from the water beyond the EZ. Collectively, they imply that EZ water may be more ordered and more stable than bulk water. It is understood that this conclusion deviates substantially from accepted theoretical expectations; nevertheless, the results of these studies show this feature consistently.
Two additional features are relevant to a fuller understanding of the implications:
The EZ is charged: typically, the charge is negative (Zheng et al., 2006). The “reason” the EZ is negatively charged is that as it forms, constituent water molecules lose protons, which then accumulate in the bulk water beyond. This accumulation is confirmed using two independent methods: pH-sensitive dyes and pH electrodes. Both show that the pH of the bulk water beyond the EZ is sharply diminished, indicating an excess of protons or hydronium ions. In other words, the EZ and the complementary region beyond the EZ effectively constitute a battery — negative in the EZ and positive in the region beyond. Substantial current can be drawn from this battery, confirming genuine charge separation.
The energy needed to build the EZ comes from radiant sources (Chai et al., submitted). That is, photons from the environment, including wavelengths in the UV, visible, and particularly infrared region, expand the EZ. Even incident infrared-intensity levels weak enough to cause insignificant heating (less than 1°C) expand the EZ by three to four times within five minutes. Hence, radiant energy is extremely effective in charging this battery.
The results outlined above are critically important for applications involving water. Any particle or solute suspended in water will be richly endowed with the kinds of features outlined above. Examples: (1) With ordered water surrounding each particle, the effective size of the particle exceeds the presumed size, possibly by an unexpectedly large amount. (2) With protons released by the ordered moiety, the pH of the bulk solution is likely to be lower than expected. (3) Light, especially infrared, is likely to have an impact on all suspensions. Thus, many features of particles or molecules in solution may differ substantially from commonly accepted views.
The same is true of water adjacent to extended surfaces, i.e., surfaces larger than those of particles. The region immediately adjacent to these hydrophilic surfaces will be extensively ordered, and the bulk zone beyond is likely to be amply endowed with protons. Hence, the latter will have a pH lower than anticipated. The presence of positive charge may have an unexpectedly profound effect on reactions occurring in reasonable proximity of the surface.
The EZ may constitute the long-anticipated “fourth phase” of water. This was suggested almost a century ago by the eminent physical chemist Sir Wm. Hardy, but largely forgotten over the years. The bottom line is that the role of interfacial water in all aqueous suspensions is far more profound than generally expected. Indeed, this unexpectedly profound role may open doors to numerous practical applications.
- Login to post comments
User login
Last articles










